dsDNA Quantification with the Echo 525 Liquid Handler for Miniaturized Reaction Volumes, Reduced Sample Input, and Cost Savings
Application Note: Rabia Khan, Jared Bailey, Jefferson Lai, and John Lesnick
Abstract
Precise dsDNA quantification is a requirement for many genomics workflows including synthetic biology, and next-generation sequencing. A variety of quantification kits are commercially available, with fluorescence-based kits like the AccuBlue NextGen dsDNA Quantitation Kit and the Quantifluor ONE dsDNA System dominating the market. The AccuBlue NextGen dsDNA Quantitation Kit covers the lower end of dsDNA quantification (3 ng - 5 pg) and the QuantiFluor ONE dsDNA System covers the higher range of dsDNA quantification (50 ng - 20 pg). The kit manufacturer’s recommended volumes are 200 μL. The main constraints on DNA quantification of reagent cost, hands on time, and required input DNA can be substantially mitigated by miniaturizing the reaction volumes.
The Echo 525 Liquid Handler was used to miniaturize and optimize these fluorescent-based quantification assays. The DNA quantification reaction volumes can be miniaturized up to 20-fold, reducing sample input and allowing more test samples than by manual arraying reagents, with up to 200 more reactions. With optimization and miniaturization of recommended protocols, standard curves generated are equivalent to that of the regular protocols. The precision and accuracy of the miniaturized reaction volumes for the two kits in an Echo 525 Liquid Handler-enabled workflow was verified by quantifying a library of dsDNA samples in a 384-well polypropylene microplate and cross checking the quantification data against the Qubit dsDNA (HS) Assay Kit.
The Echo 525 Liquid Handler utilizes Dynamic Fluid Analysis (DFA), which enables the system to determine fluid composition, height, and impedance in the source well. The required power needed to eject a droplet from the source well is then calculated within milliseconds. The droplet ejected from the source well into an inverted microtiter destination plate is 25 nanoliters (nL) in volume. By transferring a series of droplets large volumes can be pooled in the destination plate at a rate of up to 5 μL/sec.
Figure 1. (A) Echo 525 Acoustic Liquid Handler. (B) The Echo system transducer rapidly moves between wells on the source plate while the destination plate also moves, allowing rapid transfer from any well to any well for multiple fluid types.
Assay Optimization
The AccuBlue NextGen dsDNA Quantitation Kit and the QuantiFluor ONE dsDNA System are robust quantification kits used for a wide range of dsDNA with a combined linear range of 5pg - 50ng. Both the kits are fluorescence-based assays and follow a similar protocol. According to the kit-recommended protocols, the reagents are added to a 96-well microtiter plate up to the reaction volume of 200 μL, along with dsDNA serial dilution and unknown sample. This plate is then incubated for 5 minutes in the dark and read on a microplate fluorometer. Fluorescence reads are then computed to generate a standard curve to determine the amount of dsDNA present in the unknown dsDNA sample.
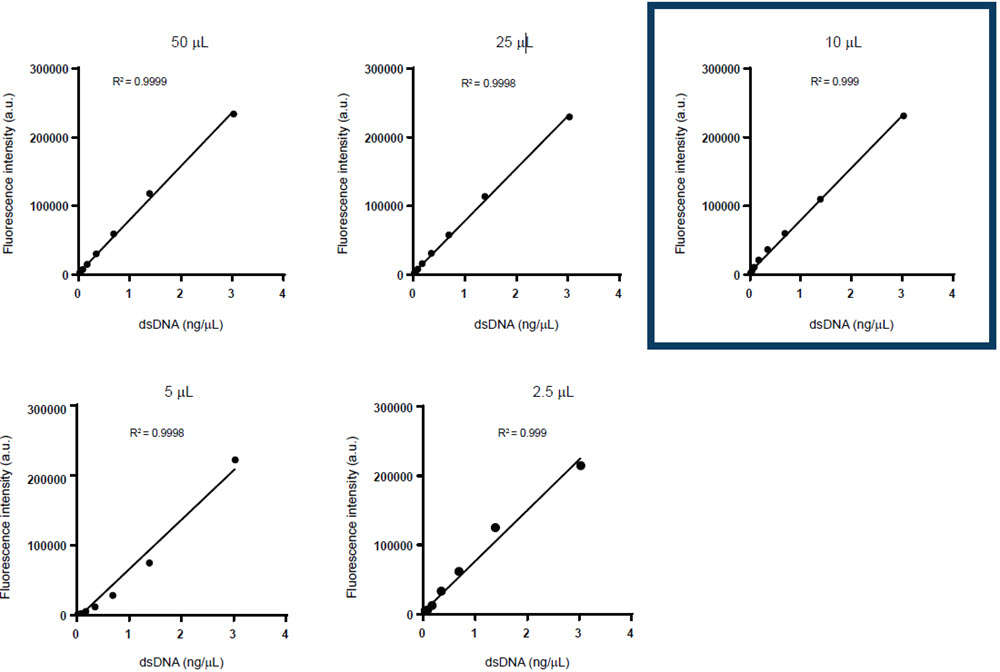
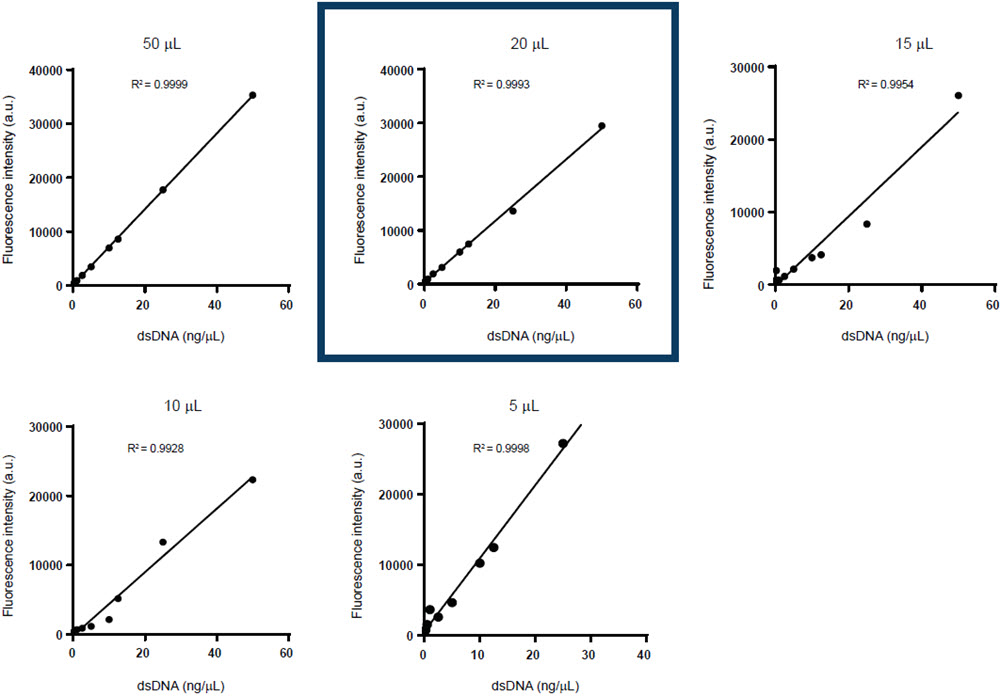
To identify the level of assay miniaturization, both the AccuBlue NextGen dsDNA Quantitation Kit and the QuantiFluor ONE dsDNA System were used to generate standard curves at variable volumes. The volumes chosen for acoustic miniaturization were 50 μL, 25 μL, 10 μL, 5 μL, and 2.5 μL for the AccuBlue NextGen dsDNA Quantitation Kit and 50 μL, 25 μL, 15 μLs, 10 μL, and 5 μL for the QuantiFluor ONE dsDNA System.
The kit’s reagents were thawed, equilibrated to room temperature and vortexed to ensure uniform mixing prior to being arrayed in an Echo Qualified 384-well source plate. Standard dsDNA dilutions were prepared afresh with the kit provided dsDNA. For the AccuBlue NextGen dsDNA Quantitation Kit, the working solution was made with AccuBlue NextGen Buffer, AccuBlue NextGen Dye (1:400), and AccuBlue NextGen Enhancer (1:100). The QuantiFluor ONE dsDNA System has only 2 components; fluorescein-based dye and standard dsDNA. A dilution was prepared with standard stock dsDNA for both the kits. The Echo 525 Liquid Handler was used to dispense the reagents and direct dilute standards into the assay plate to generate equivolume reaction mixtures with varying concentrations of dsDNA. The final reaction volumes were incubated for 5 minutes in the assay plates prior to being read on a BMG PHERAstar microplate fluorometer.
A. AccuBlue NextGen dsDNA Quantitation Kit
B. QuantiFluor ONE dsDNA System
Figure 2. Comparison of multiple standard curves generated via the Echo 525 Liquid Handler for (A) the AccuBlue NextGen dsDNA Quantitation Kit with reaction volume miniaturized up to 4-fold (50 μL), 8-fold(25 μL), 20-fold (10 μL), 40-fold (5 μL), and 80-fold (2.5 μL), and (B) the QuantiFluor ONE dsDNA System with reaction volume miniaturized up to 4-fold (50 μL), 10-fold (20 μL), 13-fold (15 μL), 20-fold (10 μL), and 40-fold (5 μL).
Assay Validation
As shown in Figure 2, each of the reaction volumes in both the kits tested in the initial miniaturization displayed a high level of linearity. However, the reactions at 10 μL for the AccuBlue NextGen dsDNA Quantitation Kit and 20 μL for the QuantiFluor ONE dsDNA System and above had the most consistent R2 value. For this reason, these volumes were chosen to be the reaction volume of choice for dsDNA quantification in an Echo 525 system-incorporated workflow.
1. AccuBlue NextGen dsDNA Quantitation Kit
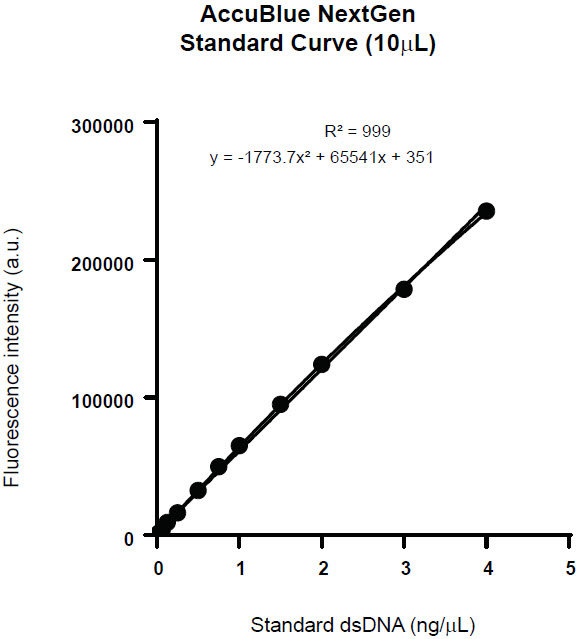
A 10 μL reaction volume enabled us to extend the linear range beyond the AccuBlue NextGen kit-recommended linearity, without compromising the R² value. The extended standard curve also enabled us to quantify a broader range of unknown dsDNA samples. Beyond 5 ng we began to lose linearity, so the recommended extended range in an Echo 525 system-incorporated workflow is 4 ng – 0.0025 ng. The kit recommends 10 ng/μL stock dsDNA to generate the standard curve. This range can be achieved by using two stock dsDNA dilutions, 2 ng/μL and 0.1 ng/μL.
In 384-well PS black microtiter plates 10 μL reaction volumes were dispensed in quadruplicate via the Echo 525 Liquid Handler, incubated for 5 minutes in the dark, and read on the PHERAstar fluorometer. The average fluorescence intensity of each reaction, standard deviation, and CV were calculated (Table 1) to generate a standard curve (Figure 3). The miniaturized reaction standard curve displays the linearity and R² values comparable to the standard curve values provided by the kit.
| Source dsDNA (ng/ μL) | Source Vol.(μL) | Buffer (μL) | Enhancer (μL) | Dye(μL) | Reaction volume (μL) | dsDNA (ng) | Average FI Counts | StDev FI Counts | % CV |
| 2 | 2 | 7.875 | 0.1 | 0.025 | 10 | 4 | 235320.3 | 3991.9 | 1.7 |
| 2 | 1.5 | 8.375 | 0.1 | 0.025 | 10 | 3 | 178717.6 | 3958.1 | 2.2 |
| 2 | 1 | 8.875 | 0.1 | 0.025 | 10 | 2 | 124089.8 | 3326.9 | 2.7 |
| 2 | 0.75 | 9.125 | 0.1 | 0.025 | 10 | 1.5 | 95020.3 | 1735.6 | 1.8 |
| 2 | 0.5 | 9.375 | 0.1 | 0.025 | 10 | 1 | 65221.8 | 1849.2 | 2.8 |
| 2 | 0.375 | 9.5 | 0.1 | 0.025 | 10 | 0.75 | 49895.6 | 2013.4 | 4 |
| 2 | 0.25 | 9.625 | 0.1 | 0.025 | 10 | 0.5 | 32683.3 | 1772.6 | 5.4 |
| 2 | 0.125 | 9.75 | 0.1 | 0.025 | 10 | 0.25 | 16342.8 | 886.5 | 5.4 |
| 2 | 0.075 | 9.8 | 0.1 | 0.025 | 10 | 0.125 | 9377.6 | 203.2 | 2.2 |
| 0.1 | 0.625 | 9.25 | 0.1 | 0.025 | 10 | 0.0625 | 3615.8 | 172.3 | 4.8 |
| 0.1 | 0.325 | 9.55 | 0.1 | 0.025 | 10 | 0.0313 | 1910.8 | 89.6 | 4.7 |
| 0.1 | 0.15 | 9.725 | 0.1 | 0.025 | 10 | 0.015 | 996.1 | 38.4 | 3.9 |
| 0.1 | 0.075 | 9.8 | 0.1 | 0.025 | 10 | 0.0075 | 685.6 | 25 | 3.6 |
| 0.1 | 0.05 | 9.825 | 0.1 | 0.025 | 10 | 0.005 | 544.3 | 23.5 | 4.3 |
| 0.1 | 0.0375 | 9.8375 | 0.1 | 0.025 | 10 | 0.0038 | 529.3 | 26.3 | 5 |
| 0.1 | 0.025 | 9.85 | 0.1 | 0.025 | 10 | 0.0025 | 453.6 | 18.8 | 4.1 |
Table 1. Protocol for preparing AccuBlue NextGen standard curve in a 10 μL reaction volume. Direct dilution is achieved by dispensing AccuBlue NextGen reagents and standard dsDNA (2 ng/uL and 0.1 ng/uL) via the Echo 525 Liquid Handler in a 384-well PS black, clear bottom microtiter plate.
Figure 3. Standard curve created from the AccuBlue NextGen dsDNA Quantitation Kit in a 10 μL reaction volume (4ng – 5 pg) with R2 value of 0.999.
2. QuantiFluor ONE dsDNA System
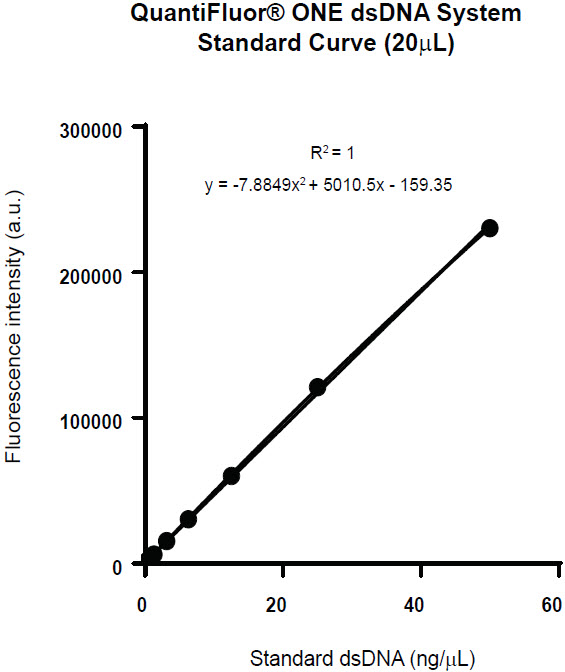
A range of 50 ng-20 pg standard curve can be generated by utilizing 12.5 ng/uL and 1 ng/uL standard stock dilutions in a 20 μL reaction volume. This encompasses a wide range for dsDNA quantification without compromising R² value. A 20 μL reaction volume was dispensed via the Echo 525 Liquid Handler into a 384-well PS black microtiter plate, incubated for 5 minutes in the dark, and read on a PHERAstar fluorometer. Average fluorescence intensity of each reaction, standard deviation and CV were calculated (Table 2) to generate a standard curve (Figure 4). The standard curve from these miniaturized reaction volumes displays the linearity and R² values comparable to the standard curve values provided by the kit.
| Source dsDNA (ng/μL) | Source Vol (μL) | Buffer (μL) | Dye(μL) | Reaction volume (μL) | dsDNA (ng/μL) | Average FI Counts | StDev FI Counts | % CV |
| 12.5 | 4 | 0 | 0.025 | 16 | 50 | 230478 | 1624.6 | 0.7 |
| 12.5 | 2 | 2 | 0.025 | 16 | 25 | 121160 | 2326.3 | 1.92 |
| 12.5 | 1 | 3 | 0.025 | 16 | 12.5 | 60209 | 1493.8 | 2.48 |
| 12.5 | 0.5 | 3.5 | 0.025 | 16 | 6.25 | 30305 | 1019.5 | 3.36 |
| 12.5 | 0.25 | 3.75 | 0.025 | 16 | 3.125 | 15422 | 255.8 | 1.66 |
| 12.5 | 0.1 | 3.9 | 0.025 | 16 | 1.25 | 6199 | 135.6 | 2.19 |
| 12.5 | 0.05 | 3.95 | 0.025 | 16 | 0.625 | 3173 | 82.8 | 2.61 |
| 12.5 | 0.025 | 3.975 | 0.025 | 16 | 0.3125 | 1500 | 39.4 | 2.63 |
| 1 | 0.15 | 3.85 | 0.025 | 16 | 0.15 | 598 | 63.3 | 5.48 |
| 1 | 0.075 | 3.925 | 0.025 | 16 | 0.075 | 266 | 4.8 | 1.81 |
| 1 | 0.05 | 3.95 | 0.025 | 16 | 0.05 | 182 | 3.7 | 2.06 |
| 1 | 0.025 | 3.975 | 0.025 | 16 | 0.025 | 76 | 1.8 | 2.37 |
Table 2. Protocol for preparing QuantiFluor ONE dsDNA System standard curve in a 20 μL reaction volume. Serial dilution is achieved by dispensing QuantiFluor ONE reagents and standard dsDNA (12.5 ng/μL and 1 ng/μL) via the Echo 525 Liquid Handler in a 384-well PS black, clear bottom microtiter plate.
Figure 4. Standard curve created from the QuantiFluor One dsDNA Quantitation Kit in a 20 μL reaction volume (50 ng – 20 pg) with R² value of 1.
Assay Verification dsDNA Quantification
In an Echo system-compatible workflow, the same protocol was followed with miniaturized volumes. Reagents were dispensed in quadruplicate by the Echo 525 Liquid Handler to 384-well black microtiter plates with a range of final volumes, incubated for 5 minutes in the dark and read on the PHERAstar fluorometer. Average fluorescence intensity of each reaction, standard deviation, and CV were calculated to generate a standard curve. The miniaturized reaction standard curve displays linearity and R² values comparable to the standard curve values provided by the kit.
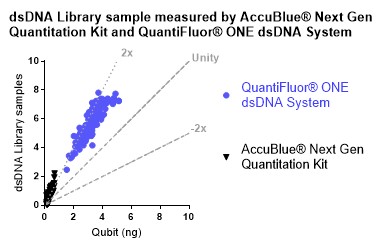
With the help of the Echo 525 Liquid Handler, a library of dsDNA assembled in a 384-well PP plate was quantified in miniaturized volumes by the AccuBlue NextGen dsDNA Quantitation Kit and the QuantiFluor ONE dsDNA System. The above mentioned miniaturized and optimized workflow was adapted for this test. The Echo Plate Reformat Application was used to dispense kit reagents and samples into 384-well PS black microtiter plates followed by a 5-minute incubation and reading the plate on the PHERAstar fluorometer. A second-order polynomial equation was derived from the standard curve, and fluorescence counts were then converted from arbitrary units to ng of dsDNA. The acquired data was plotted against the values of the same dsDNA libraries quantified by the Qubit dsDNA (HS) Assay Kit. The graph in Figure 5 displays a comparable correlation between Qubit values and the ones generated from the 2 kits mentioned above.
Figure 5: dsDNA NGS library quantified by the AccuBlue NextGen dsDNA Quantitation Kit and the QuantiFluor ONE dsDNA System and plotted against the values of the same dsDNA libraries quantified by the Qubit dsDNA (HS) Assay Kit.
Conclusion
The AccuBlue NextGen dsDNA Quantitation Kit and the QuantiFluor ONE dsDNA System can be miniaturized up to 20-fold using the Echo 525 Liquid Handler. Kit reagents can be dispensed in miniaturized volumes using the Echo Plate Reformat Application into a destination plate.
Standard curves generated for both kits in miniaturized reaction volumes display R² values comparable to the kit-recommended protocols, which can be used to determine the amount of dsDNA present in a sample volume as low as 0.025 μL.
These kits can be effectively used to quantify dsDNA library samples via the Echo 525 Liquid Handler. This can mitigate the constraints on DNA quantification of reagent cost, hands-on time, and required input DNA, and be highly beneficial for applications such as synthetic biology, NGS library prep, or quantifying precious low concentrations of unknown dsDNA samples.
| Equipment | Manufacturer | |
| Echo 525 Liquid Handler | Beckman Coulter Life Sciences | |
| Allegra X-14 Centrifuge | Beckman Coulter Life Sciences | |
| BMG PHERAstar | BMG Labtech | |
| Qubit | ThermoFisher | |
| Materials for AccuBlue NextGen dsDNA Quantitation Kit Biotium #31060 | Kit Recommended | 10-Jan |
| Reaction volume | 200 μL | 10 μL |
| AccuBlue NextGen Dye, 400X | 0.5 μL | 0.025 μL |
| AccuBlue NextGen Enhancer, 100X | 2 μL | 0.1 μL |
| AccuBlue NextGen Buffer, 1X | 197.5 μL | >~9 μL |
| High Sensitivity DNA Standard | 10 μL | (0.025-~2) μL |
| Reactions per kit | 1250 | 250,000 |
| Materials for QuantiFluor ONE dsDNA System Promega #E4871 | Kit Recommended | 20-Jan |
| Reaction volume | 200 μL | 20 μL |
| AccuBlue NextGen Dye, 400X | 200 μL | 16 μL |
| High Sensitivity DNA Standard | 1 μL | (4-0.025) μL |
| Consumables | Manufacturer | Part Number |
| 384-well PP Microplate | Beckman Coulter Life Sciences | 001-14555 |
| 384-well LDV Microplate | Beckman Coulter Life Sciences | 001-12782 |
| 384-well Black Clear-Bottom Microplate | Greiner | #781096 |
| 384-well Black μClear Microplate, Small Vol, LoBase | Greiner | #788096 |
| TapeStation Plate | Agilent | #5067-5150 |
| Qubit dsDNA HS Assay Kit / Qubit Microtube | ThermoFisher | #Q32851 / #Q32856 |
Biomek Automated Workstations and Echo Liquid Handlers are not intended or validated for use in the diagnosis of disease or other conditions.